
Technologies
Provide professional solutions for each problem

Easy 3D
Super easy, super resolution
The patented single-beam light path of our EASY3D module allows you to capture the complete three-dimensional structure of your sample features. Furthermore, EASY3D is compatible with any moderate- to the high-NA objective lens and can correct aberrations in the STED beam, giving you complete freedom to embed and image your sample in the best possible way.
EASY 3D
Revolutionary SLM
The best helper for imaging
The EASY3D device uses a programmable spatial light modulator (SLM) to create phase patterns required for 2D and 3D STED microscopy. At the same time, it can also be used to correct optical aberrations of the STED beam.
The SLM allows a revolutionary design with only one STED beam instead of two separate STED beams for lateral (XY) and axial (Z) resolution enhancement, respectively. The resolution enhancement can be tuned between pure XY and mainly Z enhancement. The single-beam design obsoletes any beam recombination and alignment resulting in outstanding system stability.
EASY 3D
Bright Images with
Optical Aberration Control
EASY 3D
Single Beam Design, no need for alignment, forever

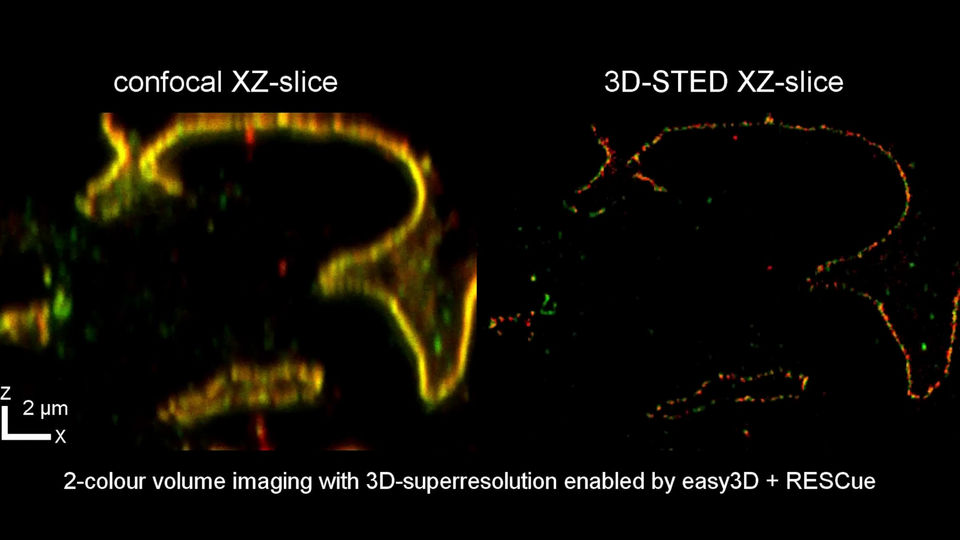
The EASY3D design uses only a single beam to carry XYZ-STED. That’s why it’s always in perfect alignment.

In a conventional 3D STED design, one beam carries XY-STED and the other Z-STED. Both beams can drift apart, leading to misalignment and loss of signal and resolution.
EASY 3D
Switch Between Lenses on the fly: Oil Immersion, Water Immersion, Silicone Oil Immersion…

When using an oil immersion lens to image a layer of fluorescent beads through 13 μm of water, the image suffers from a significant loss in fluorescence intensity. This results in unusable images, both for confocal and STED imaging.

The EASY 3D design allows you to change objectives during experiments and use the most suitable lens in STED mode. Additionally, you can further correct for aberrations in all beams with our adaptive optics.
EASY 3D
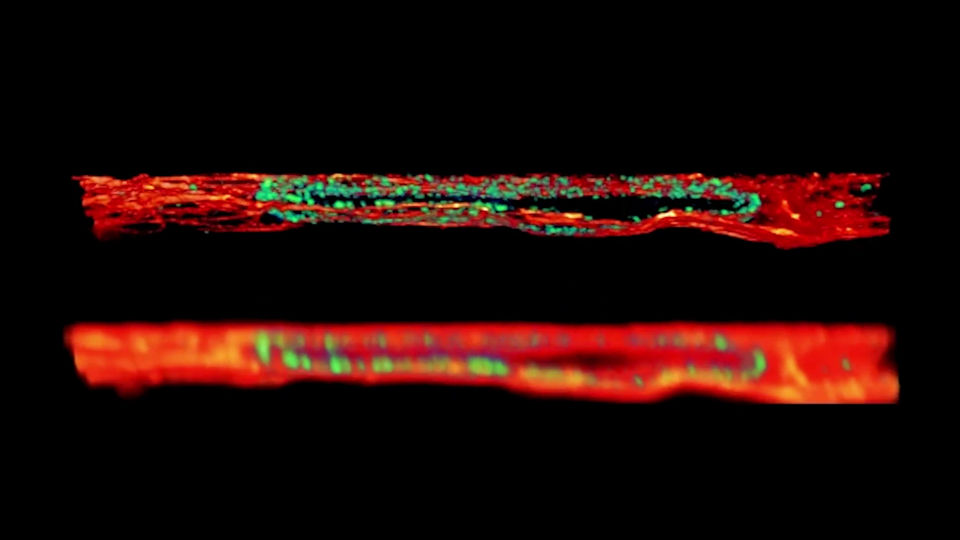
Thick Tissue, no problem from now on

Abberiors Spatial Light Modulator (SLM)

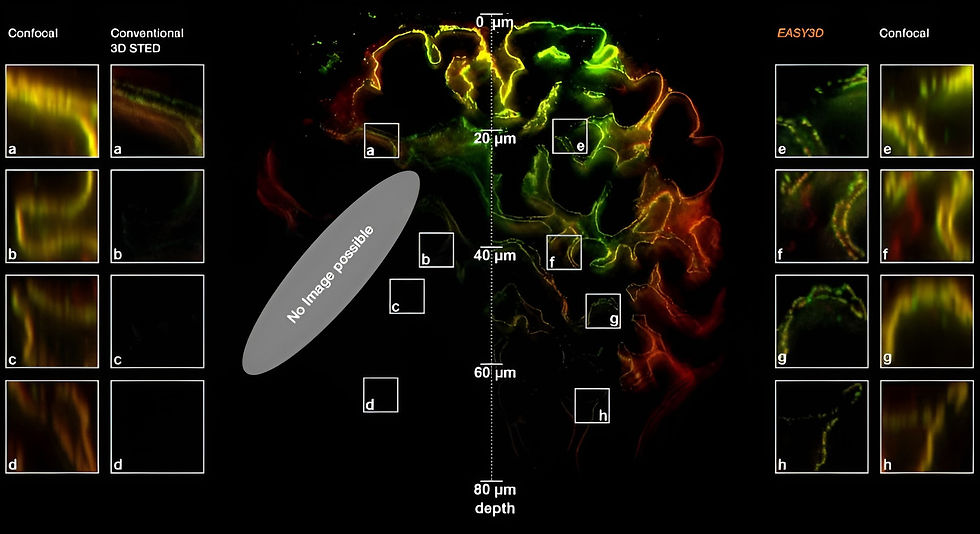
Deep 3D STED Resolution down to 180µm
With our programmable spatial light modulator (SLM), the aberration correction allows you to image with 3D STED resolution down to 180 µm into the sample. This is only possible if you can correct the Z-STED zero depending on the sample depth.

EASY 3D
The Best Assistant for Fine Imaging
-
Tune the resolution in XY and Z to fit your imaging problem
-
Switch between objective lenses on the fly: oil immersion, water immersion, silicone oil immersion…
-
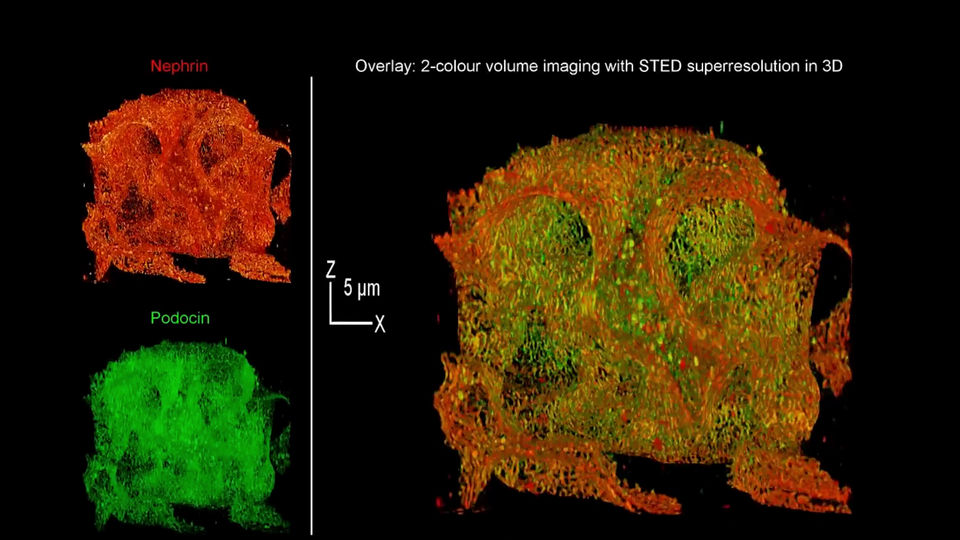
3D resolution: 90 × 90 × 90 nm minimum, 75 × 75 × 75 nm typical
-
2D resolution: Better than 30 × 30 nm
-
Aberration correction: keep the STED PSF in shape even deep inside your sample (> 20 μm)
-
Any Abberior MINFLUX and FACILITY system can be equipped with the EASY3D device

Service Name
This is a Paragraph. Click on "Edit Text" or double click on the text box to edit the content and make sure to add any relevant information that you want to share with your visitors.


Adaptive Illumination
100% resolution,
4% light dose
ADAPTIVE ILLUMINATION dramatically reduces the light dose on the sample by up to two orders of magnitude by decreasing or shutting down laser irradiation whenever it is clear that there is no structure present at the current pixel. This substantially reduces photobleaching. Often, ADAPTIVE ILLUMINATION makes all the difference between a crisp image and no superresolution image at all.
Adaptive Illumination
What is Adaptive Illumination
THE ADAPTIVE ILLUMINATION package consists of three related but different techniques, RESCUE, DYMIN, and MINFIELD. What they have in common is that for every pixel during the scan, they dynamically adapt the amount of laser light to the underlying structure of your sample instead of aimlessly using the same laser power everywhere. Dark or out-of-focus areas of the image experience almost no irradiation, while regions, where fluorescent markers are present and in focus are imaged with 100% signal and resolution. This means light is put only where it has the maximal effect and nowhere else.
ADAPTIVE ILLUMINATION substantially reduces photobleaching and enables long-term measurements over volumes or dozens of frames, whereas conventional STED has long bleached the sample. Therefore, it is the number one choice for reducing the light dose on your delicate specimen.

RESCUE
RESCUE
Solving Problems with Live Cell Imaging
For every pixel during the scan, RESCUE (Reduction of State transition Cycles) shortly probes if there is any structure. And if not, illumination is immediately shut down. This quick probing is done in confocal mode typically and for a time period of about 10% of the total pixel dwell time (“confocal probing”). Only if the signal is detected during this probing step, illumination and detection carry on for the remainder of the pixel dwell time.
In a typical sample, where 80% of the areas are dark, RESCUE alone reduces the amount of STED photons experienced by the specimen by 80% and the number of excitation photons by 70%, without any loss of resolution or signal whatsoever. Combine this with DYMIN and our use of pulsed instead of continuous-wave lasers, and you dramatically reduce light dose down to a few percent. This does matter for live-cell and volume imaging.
RESCUE
Amplified Volume Imaging with RESCUE
When recording a volume, i.e., a stack of 2D images, the effect is even more dramatic because the STED-donut (being diffraction limited) has a size along the z-direction much larger than the thickness of the individual images. Thus, recording one image means that the STED light is also applied to up to twenty others without benefit! Because of this, the gains achieved with RESCUE are amplified up to 20-fold for volume imaging.




DYMIN
Even Better Probing with
DYMIN
After RESCUE, DYMIN (Dynamic Minimum) further reduces sample areas where maximum STED power is applied. This is achieved by probing for structure not only with the excitation laser but also with a small amount of STED light in a multi-step fashion. As with RESCUE, illumination is shut off when no structure is detected in the first step. But if the structure is present, a little STED power is applied next to increase the resolution, thus sharpening the probing. The trick is that small STED powers quickly yield a relatively significant increase in resolution, which means that with little additional irradiation, a lot can be learned about the precise location of the fluorescent structures. The full resolution power is applied only if successive increases in STED power confirm that the scan currently targets sample structures; otherwise, light is immediately shut off for the rest of the pixel time.
This multi-step probing at increasing resolutions reduces the overall bleaching by orders of magnitude. The gains can be invested in either dramatically more signal or significant improvements in optical resolution.

DYMIN greatly expands your possibilities

DYMIN enables long-term measurements
DYMIN
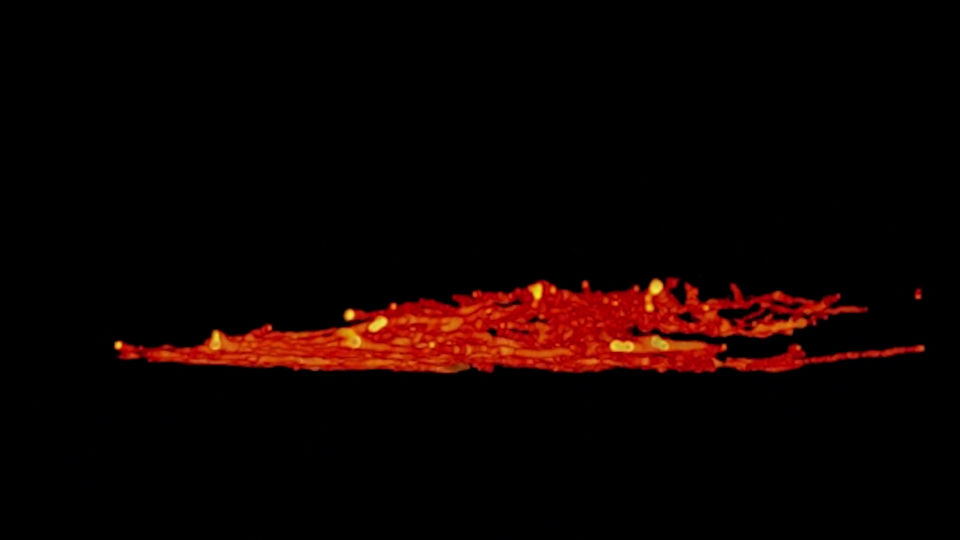
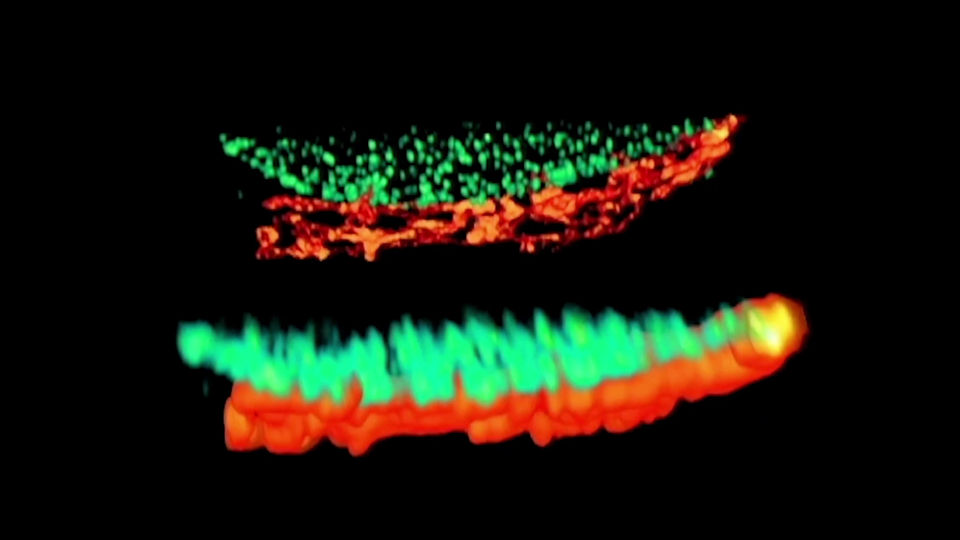
DYMIN Imaging
A 3D image stack of the mammalian nucleus was recorded with EASY3D and DYMIN, and conventional STED microscopy. Shown are xz sections of the stack and a confocal image after the stack acquisition, highlighting the remarkable bleaching reduction of DYMIN. Note that DYMIN enabled us to acquire the complete stack with superior resolution and signal. Shown are Vero cells labeled with antibodies against nuclear pore complex protein (nup153).

3D Stacks with and without DYMIN

Confocal image taken after a volume recording without and with DYMIN
DYMIN

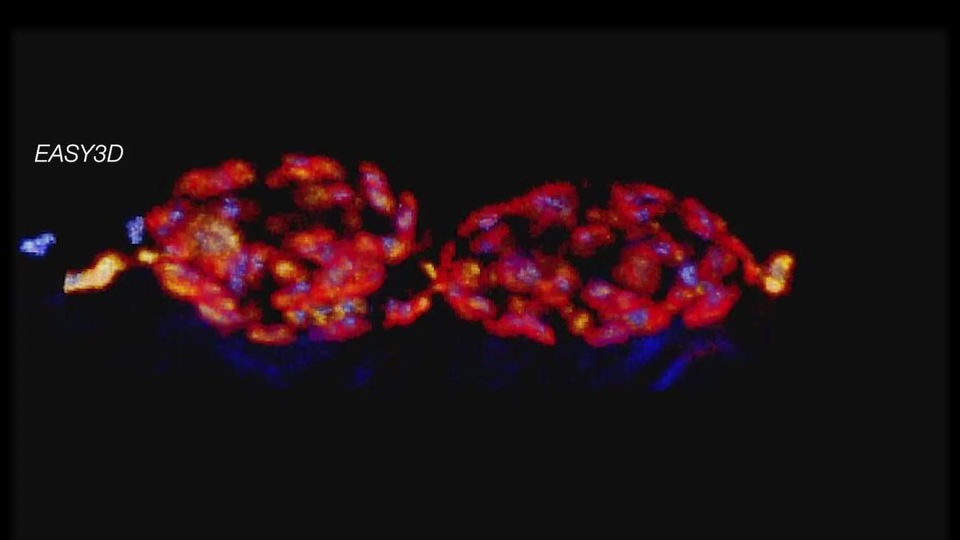
DYMIN Imaging
DYMIN very clearly resolves spectrin rings with an extraordinary signal-to-noise ratio. Primary hippocampal neurons (22 days in vitro) show the characteristic ~ 192 nm βII spectrin periodicity along distal axons.
Dye: Abberior STAR 635P, Excitation: 635 nm, STED: 775 nm. Sample with courtesy from Elisa D’Este (MPIbpc).
MINFIELD
MINFIELD
STED without STED photons
MINFIELD is yet another way to put light only where it’s needed in your sample. MINFIELD avoids scanning the STED-donut over the field of view entirely, instead only scanning the low-power central hole of the STED-donut over the fluorescent structure. Obviously, the imaged region has to be smaller than the diffraction-limited hole in the donut, but the result there is a resolution that is unmatched in the STED world.


MINFIELD
2D MINFIELD
MINFIELD presented on human immunodeficiency virus type 1 (HIV-1). Confocal and MINFIELD images with a field size of 160 nm. Images taken from J. Hanne et. al.”Stimulated Emission Depletion Nanoscopy Reveals Time-Course of Human Immunodeficiency Virus Proteolytic Maturation” ACS Nano 10, 8215-8222, 2016.


2D MINFIELD presented on gp210 and clathrin with both a MINFIELD size of 200 nm. Imaging examples of gp210 and clathrin.



3D MINIELD
3D MINFIELD presented on standing DNA nano-ruler with a nominal separation of 91 nm, xz-view. Confocal and MINFIELD images with a field size of 180 nm x 300 nm.
